The Discovery SDT 650 is a differential scanning calorimeter and thermogravimetric analyzer. The TGA measures change in mass as heat is applied to the chamber in a given atmosphere. The TGA’s temperature ranges from 50°C to 1500°C. Currently, there are two atmospheres provided by the Materials Characterization Lab:
- air
- nitrogen (N2)
When performing analysis of a sample, it is important to consider atmospheric conditions for the end goal of the analysis. Air may cause oxidation in some samples, which can either be included in the experiment as part of the analysis, or may be undesirable and should be avoided. Other samples may react with nitrogen, and should not be run in nitrogen-only atmospheres. In most cases, a choice can by made by considering the final goal of the TGA or DSC analysis. If you are interested in learning about whether a sample oxidizes or burns, air is necessary to facilitate both reactions. If you’re concerned that your sample may ignite, or if your final application of the material will be in an inert atmosphere, nitrogen will be a better choice.
A (failed) attempt to prove the difference between the gasses
An experiment was conducted to analyze the effects of both gases provided by the lab by forming an oxide layer in an air atmosphere and a nitride layer in an N2 atmosphere on powdered silicon. Two silicon samples of were placed in the TGA. The first sample was placed in an air atmosphere, raised to 950°C, and held at an isotherm for five hours. The second sample was placed in an N2 atmosphere, raised to 1250°C, and held at an isotherm for five hours. It was hypothesized that, in these conditions, the sample in an air atmosphere should form a silicon oxide layer, and in the N2 atmosphere should form a silicon nitride layer.
Through analysis of the results, it was apparent that both samples gained mass throughout the experiment, hinting the hypothesis was correct. The TGA results are provided in figures 1 and 2 for the air and N2 atmospheres respectively. Both graphs depict weight percent as the y-axis and time (with temperature markings) as the x-axis. The samples were further analyzed in an SEM using its EDS capabilities, to confirm the hypothesis. To much surprise, neither sample contained significant nitrogen. Both samples contained silicon and oxygen. These results were expected for the air atmosphere sample but not for the N2 atmosphere sample. Both samples had been placed in an aluminum oxide crucible for the experiment, and it seems as though the heating program replicated sintering; the aluminum atoms in the crucible swapped places with silicon allowing for silicon oxide molecules to form no matter the atmosphere.
Figure 1: Graph depicting TGA results of a silicon sample placed in an air atmosphere, raised to 950°C, and held at this temperature for five hours.
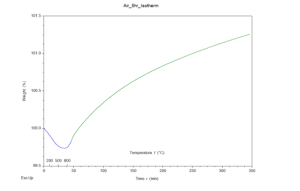
Figure 2: Graph depicting TGA results of a silicon sample placed in an N2 atmosphere, raised to 1250°C, and held at this temperature for five hours.
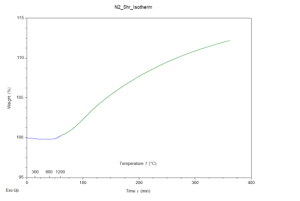
Figure 3: SEM results using air (top) and N2 (bottom) atmospheres


Written by Parker Mancuso
